





Related Attributes
Product details
Levofloxacin is the left isomer of Ofloxacin, which is 8 times more water-soluble than Ofloxacin, easier to be made into injections, and has fewer toxic side effects. It was firstly marketed in Japan in 1994, and belongs to the third-generation of quinolone antimicrobial drugs.
It is a third generation quinolone antibacterial drug. It mainly exerts antibacterial effects by inhibiting topoisomerase IV and DNA rotase (topoisomerase II), which are required for bacterial DNA replication, transcription, repair and recombination.
Levofloxacin is twice as active as ofloxacin against most clinical isolates.
Because of its good activity against common respiratory pathogens Streptococcus pneumoniae, Haemophilus influenzae, and Mycoplasma pneumoniae, it is also known as & ldquo; respiratory quinolone & rdquo;, and its antibacterial activity against Pseudomonas aeruginosa is stronger than that of its predecessor.
Because of its broad antibacterial spectrum, strong antibacterial effect, no need to do skin sensitivity test and other advantages, it has been widely used in the clinic.
However, long-term clinical practice has shown that levofloxacin causes a wide range of adverse reactions (ADRs).
In the 2016 National Annual Report on Adverse Drug Reaction Monitoring, the top ranked drugs were anti-infective drugs, and levofloxacin ranked as the top anti-infective drug.
As a result, its use in the clinic has been somewhat limited.

Uses and functions of Levofloxacin.
Levofloxacin has excellent in vitro activity, less toxic side effects than ofloxacin, greater safety and good pharmacokinetic properties.
It is a broad-spectrum fluoroquinolone antibacterial drug for oral or parenteral use, which can be widely used in a variety of bacterial infections, such as respiratory tract infections, gynaecological infections, skin and soft tissue infections, surgical infections, biliary tract infections, sexually transmitted diseases, and otorhinolaryngological infections.
Mainly used in the manufacture of various types of levofloxacin capsules, tablets and other antibacterial preparations
It is a fully synthetic antibacterial agent used for the treatment of respiratory tract, urinary tract and skin tissue infections, etc.

Drug interactions of Levofloxacin.
1, and non-steroidal anti-inflammatory drugs at the same time, there is the possibility of triggering convulsions.
2、Combined with theophyllines, it may lead to the increase of theophylline blood concentration and the symptoms of theophylline poisoning.
3, and containing magnesium or aluminium antacids, aluminium sulfate, metal cations (such as iron), zinc-containing multivitamin preparations and other drugs can significantly reduce the absorption of levofloxacin.
4、Combined with hypoglycaemic drugs, should pay attention to the monitoring of blood glucose concentration in the process of using drugs.

Product Method of Bulk Levofloxacin Powder.
Method 1: Racemic ofloxacin is directly split by high performance liquid chromatography column filled with special stationary phase to obtain the levulinic acid; or oxfloxacin is treated with hydroxylamine sulphate, acidified with hydrochloric acid to obtain the hydrochloride salt by alkaline ion-exchange column, the amphoteric compounds are obtained, and added with (S)-(+)-mandelic acid, which forms crystals with the salt of the (-)-isomer, and then passes through the ion-exchange resin, and then reduced to deaminate the product. The product was obtained.
Method 2: Using 2,3,4,5-tetrafluorobenzoic acid as raw material, ethyl 2-(ethoxymethylene)-3-oxo-3-(2,3,4,5-tetrafluorophenyl)propanoate is prepared by the commonly used method, and then the asymmetric carbon atoms are introduced by reacting with (S)-2-aminopropanol, and the product is obtained by closing the ring, hydrolysing and introducing methyl piperazine.
Method 3: 2,3-difluoro-6-nitrophenol can also be used as a raw material and reacted with glycidyl p-toluenesulfonate in R configuration in the presence of a phase transfer catalyst to produce an optically active compound, which is then reduced and condensed with diethyl ethyl ethoxymethylenemalonate (EMME), and then treated with Mitsunobu's reagent, ring closure, ring closure, hydrolysis, and introduction of piperazinyl groups to obtain the product.
WHY CHOOES US?

OUR CERTIFICATE

CUSTOM PROCESS

OUR PACKAGE

OUR EXHIBITION

OUR FACTORY

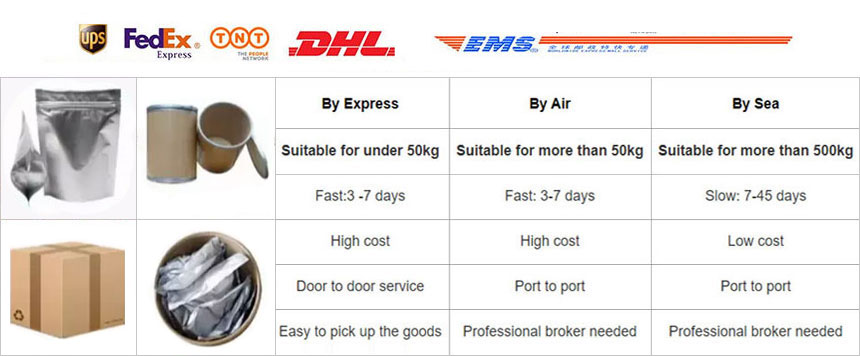
Shipping
Pharmaceutical Intermediate manufacturers
©2022 Xi'an Henrikang Biotech Co., Ltd.,