Pharmaceutical Excipients Sucrose Powder
Related Attributes
Product details
Introduction to the Sucrose of Pharmaceutical Excipients:Sugar has been around for thousands of years. It is the main product of photosynthesis and widely distributed in plants, especially in sugar beet, sugarcane and fruit. The sugar with sucrose as the main component can be divided into rock sugar, white granulated sugar, white sugar and brown granulated sugar (also known as brown sugar or black sugar) according to its purity from high to low. Sucrose is the most abundant in beet and sugarcane, and the white sugar and brown sugar used in ordinary times are sucrose.
Chemical sucrose, organic compound, molecular weight 342.3. Colorless crystal with optical rotation but no variable rotation. The molecular formula of sucrose is C12H22O11. Sucrose is easily hydrolyzed by acid, which produces equal amounts of D-glucose and D-fructose. It's not reductive. The fermented caramel can be used as a color enhancer for soy sauce.
Sucrose, the main product of photosynthesis, is widely distributed in plants, especially in sugar beet, sugarcane and fruit. Sucrose is the main form by which plants store, accumulate and transport sugar. Usually eat white sugar, brown sugar is sucrose. Sucrose is formed by the dehydration and condensation of one molecule of glucose and one molecule of fructose. It is more soluble in water than in ethanol and is second only to fructose in sweetness.Physical properties of sucrose:Sucrose is a white, sweet solid. Solubility: Easily soluble in water, aniline, azobenzene, ethyl acetate, alcohol and water mixture. Insoluble in gasoline, petroleum, anhydrous alcohol, CHCl3, CCl4[1]
Solubility in water: 2.1g of sucrose can be dissolved per gram of water, i.e. solubility is 210g(25℃). It's a high solubility sugar.
Melting point: 186℃
Heat: 17 kilojoules per gram
Note that the silver mirror reaction cannot occur because sucrose (a structure that does not contain hemiacetals (ketones)) is not reducing. But the silver mirror reaction can occur after hydrolysis.
The main raw materials for sucrose are sugar cane (Saccharum spp.) and sugar beet (Beta vulgaris). The sugar cane or beet is crushed by a machine, the juice is collected, filtered and treated with lime to remove impurities, and then bleached with sulfur dioxide. Boil the treated sugar juice, drain the bottom of the impurities, scrape the foam that rises to the surface, then remove the heat and allow the syrup to crystallize into sucrose. Sucrose is broken down into fructose and glucose by the digestive juices in the body's messaging system and absorbed by the small intestine. Sucrose is thought to cause some health problems, the most common of which is tooth decay, because bacteria in the mouth can convert sucrose from food into acid, which can erode tooth enamel. Sucrose is high in calories and excessive intake is likely to cause obesity.
WHY CHOOES US?

OUR CERTIFICATE

CUSTOM PROCESS

OUR PACKAGE

OUR EXHIBITION

OUR FACTORY

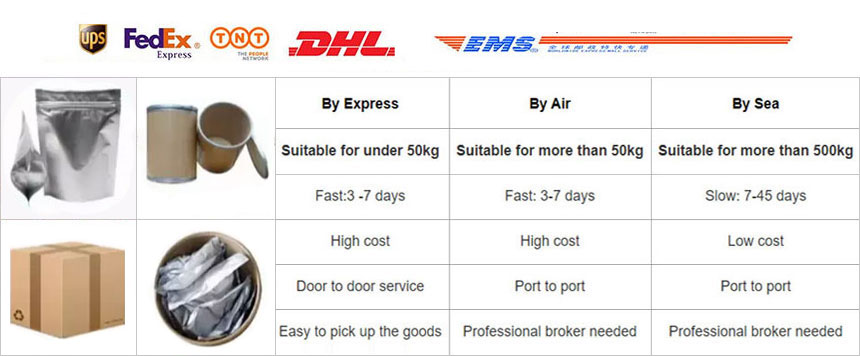
Shipping