







Related Attributes
Product details
Daptomycin Powder is a new structured cyclic peptide antibiotic derived from the fermentation broth of S. reseosporus, Antibiotics Daptomycin Powder is used in the treatment of severe skin infections and Antibiotics Daptomycin Powder is used in infectious diseases. is used in the treatment of severe skin infections and Antibiotics Daptomycin Powder is used in infectious diseases. Daptomycin Raw Materials is a pharmaceutical intermediate for the treatment of complicated skin and soft tissue infections caused by certain Gram-positive bacteria. Daptomycin Raw Materials is a pharmaceutical intermediate for the treatment of complicated skin and skin structure infections caused by some Gram-positive sensitive strains of bacteria.

Uses and functions of Daptomycin Powder.
It is used for the treatment of endocarditis, septicaemia, peritonitis and urinary tract infections caused by staphylococci, streptococci and enterococci, especially endocarditis caused by resistant strains. Animal studies have shown that the use of daptomycin for the treatment of endocarditis, peritonitis, pneumonia and myelitis caused by MRSA or other drug-resistant strains is superior or equal to vancomycin, and that daptomycin has a long t1/2 in vivo and fewer toxic side effects, making it of considerable clinical use.

The first case of failure of daptomycin in the treatment of endocarditis caused by a resistant strain of Staphylococcus aureus was reported in October 1990. The reasons for this were estimated to be the high affinity of daptomycin for the protein (90%), the low dose administered and the different pharmacokinetic parameters of the patient compared to healthy subjects.
Pharmacological Effects of Daptomycin Powder.
Daptomycin was first approved by the US Food and Drug Administration in September 2003 for the treatment of severe skin infections.
Approved for use in infectious diseases in March 2006.
Approved by the European Commission in January 2006 for the treatment of complicated skin and soft tissue infections caused by certain gram-positive bacteria.

On 6 September 2007 Cubist Pharmaceuticals announced that the European Union has approved its antibacterial drug daptomycin for injection (Cubicin) for the treatment of right heart infective endocarditis caused by Staphylococcus aureus and Staphylococcus aureus bacteraemia associated with right heart infective endocarditis or complicated skin and soft tissue infections.
On July 29, 2010, the U.S. Food and Drug Administration (FDA) issued an advisory to patients and healthcare professionals regarding the use of the intravenous drug daptomycin (Cubist Pharmaceuticals, Inc., trade name Cubicin) during medical treatment as a possible cause of eosinophilic pneumonia.
Eosinophilic pneumonia is a rare and very serious disease whose symptoms include fever, cough, shortness of breath and difficulty in breathing.
Daptomycin is also known as daptomycin.
Production method of Daptomycin Powder.
Daptomycin is a new structural cyclic peptide antibiotic derived from the fermentation broth of S. reseosporus, discovered by Eli Lilly and Company in the 1980s.
It has a novel chemical structure and a mode of action unlike any other approved antibiotic: it disrupts the biosynthesis of peptidoglycan in the bacterial cell wall by disrupting the transport of amino acids through the cell membrane, thereby altering the properties of the cytoplasmic membrane, disrupting bacterial cell membrane function in several ways, and rapidly killing Gram-positive bacteria. Daptomycin

In addition to its ability to act on most clinically relevant Gram-positive bacteria, daptomycin is important because of its potent in vitro activity against isolates that have shown resistance to methicillin, vancomycin and linezolid, a property of great clinical importance in patients with critical infections.
Daptomycin has a synergistic effect when combined with nethicillin, amikacin, imipenem and fosfomycin to increase antibacterial activity, and has excellent antibacterial activity against SmaGmrBla-enterococci when combined with teicoplanin and vancomycin, and daptomycin has a synergistic effect with gentamicin against glycopeptide antibiotic-resistant Streptococcus faecalis.
This has been demonstrated in animal models. When combined with tobramycin, daptomycin attenuates the nephrotoxicity of tobramycin, which is the opposite of vancomycin.
WHY CHOOES US?

OUR CERTIFICATE

CUSTOM PROCESS

OUR PACKAGE

OUR EXHIBITION

OUR FACTORY

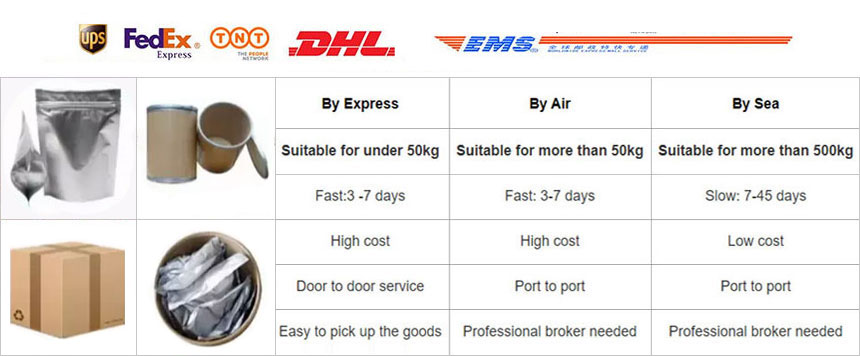
Shipping
Pharmaceutical Intermediate manufacturers
©2022 Xi'an Henrikang Biotech Co., Ltd.,